electron configuration fe3+|electron configuration for nitrogen : iloilo In order to write the Mg electron configuration we first need to know the . Football live scores page on Flashscore.com offers all the latest football results from more than 1000+ football leagues all around the world including EPL, LaLiga, Serie A, Bundesliga, UEFA Champions League and more. Find all today's/tonight's football scores on Flashscore.com.
electron configuration fe3+,Electron Configuration for Iron (Fe, Fe2+, and Fe3+) Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period .Therefore the Calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 .
electron configuration for nitrogenSince 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .Therefore the N electron configuration will be 1s 2 2s 2 2p 3. Video: Nitrogen .In order to write the Mg electron configuration we first need to know the .Electron Configuration Notation:-shows the arrangment of electrons around the .Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 .
In order to write the Argon electron configuration we first need to know the .Therefore the O electron configuration will be 1s 2 2s 2 2p 4. Video: Oxygen .

Electron Configuration of Fe2+ and Fe3+. chemistNATE. 266K subscribers. Subscribed. 5.3K. 705K views 10 years ago. The electron configuration for Fe2+ is 1s2 2s2 2p6 . Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons occupy. Ground state means that the atom has the lowest energy .

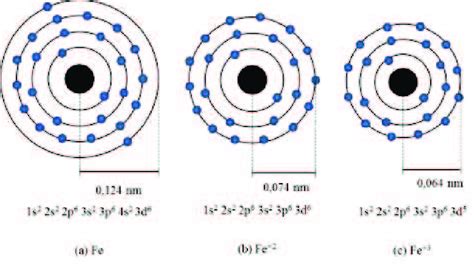
What is the electron configuration for the Fe3+ ion? The electron configuration for the Fe3+ ion is [Ar] 3d5 4s0. This is because the Fe3+ ion has lost 3 .The distribution of electrons is as 2 electrons in 1s subshell, 2 electrons in 2s subshell, 6 electrons in 2p subshell, 2 electrons in 3s, 6 electrons in 3p and 5 electrons in 3d .The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. By knowing the electron configuration of an element, we can predict and .Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss .
What are electron configurations? Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The .
The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be .March 23, 2023. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also .
So if you're thinking about the subshell, the s subshell could fit two electrons, the p subshell can fit six electrons, the d subshell can fit 10 electrons, and the f subshell can fit 14 .
electron configuration fe3+ The electron configuration for Fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6The electron configuration for Fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5Ask me questions: http://www.chem. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 5.1.3 or 5.1.4 ). Thus, the electron configuration and orbital diagram of lithium are:
The electron configuration of Fe2+ is 1s2 2s2 2p6 3s2 3p6 3d5. Fe3+ atom loses four more valence electrons from its d-orbital to attain noble gas The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). For instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.
The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels, this added stability is enough to shift an electron from one orbital to another. In heavier elements .Electronic Configuration of Iron. The chemical element iron has the atomic number 26 and the symbol Fe (from Latin: Ferrum). It’s a transition metal from group 8 of the periodic table’s first transition series. It is the most abundant element on Earth by mass, coming in second to oxygen (32.1% and 30.1%, respectively), and it makes up much . La configuration électronique des ions Fe2+ et Fe3+ est obtenue en retirant d'abord des électrons de l'orbitale 4s avant de les retirer de l'orbite 3d. La configuration électronique de Fe, Fe2+ et Fe3+ influence leurs propriétés chimiques et leur réactivité. dans le tableau périodique. Fe3+ Edelgas Elektronenkonfiguration. Wenn 3 Elektronen von einem neutralen Fe-Atom entfernt werden, wird ein Fe3+-Ion gebildet. Das Edelgas fe3+ Elektronenkonfiguration ist 1s2 2s2 2p6 3s2 3p6 3d5. Zuerst werden 1 Elektronen aus dem 2s-Orbital entfernt, da es eine höhere Energie als das 4d-Orbital hat, und dann wird 3 .Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, .If you need to write the full electron configuration for an anion, then you are just adding additional electrons and the configuration is simply continued. For example, we know that Oxygen always forms 2- ions . 2. Find your atom in the ADOMAH table. To write electron configuration of an element, locate its symbol in ADOMAH Periodic .
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 . Solution. 1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration.
The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1s, while the outermost shell ( 2s) has 1 electron. Figure 2.7.1 2.7. 1: Shell diagrams of hydrogen (H), helium (He), lithium (Li), and Berryellium (Be) atoms. (CC BY-SA 2.0 UK; Greg Robson modified by Pumbaa via Wikipedia)Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 2.6.6 2.6. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series:
electron configuration fe3+ electron configuration for nitrogen The electron configuration for the iron(III) ion is: "1s"^2"s"^2"2p"^6"3s"^2"3p"^6"3d"^5" The element iron, Fe, has the atomic number 26, which is the number of protons in its atomic nuclei. A neutral iron atom has 26 protons and 26 electrons. In order to form a 3^+ ion, it must lose three electrons. The ground state .
electron configuration fe3+|electron configuration for nitrogen
PH0 · electron configuration table
PH1 · electron configuration for silicon
PH2 · electron configuration for oxygen
PH3 · electron configuration for nitrogen
PH4 · electron configuration for lithium
PH5 · electron configuration for every element
PH6 · electron configuration for cobalt
PH7 · electron configuration for aluminum
PH8 · Iba pa